The animals that can live forever
No one likes the thought of growing old. Despite our many human endeavours to escape or delay the process of ageing, it seems to be an inevitable part of life.
But … why? Why do living things gradually fall apart when they grow older?
There is a word for it: senescence. No, it’s not the rock band who sang ‘Bring Me to Life’; senescence is the state of gradual deterioration of normal functioning. At the cellular level, it means cells stop dividing and they eventually die. It can also apply to an entire organism (where a living thing can no longer respond adequately to outside stressors), or to specific organs or tissues (like leaves dying and falling from trees in autumn).
While there are ways we can slow down (or speed up) the rate at which senescence occurs, it is still going to happen one way or another. However, a few species can escape the ageing process completely.
The ‘immortal’ jellyfish, Turritopsis dohrnii
To date, there’s only one species that has been called ‘biologically immortal’: the jellyfish Turritopsis dohrnii. These small, transparent animals hang out in oceans around the world and can turn back time by reverting to an earlier stage of their life cycle.
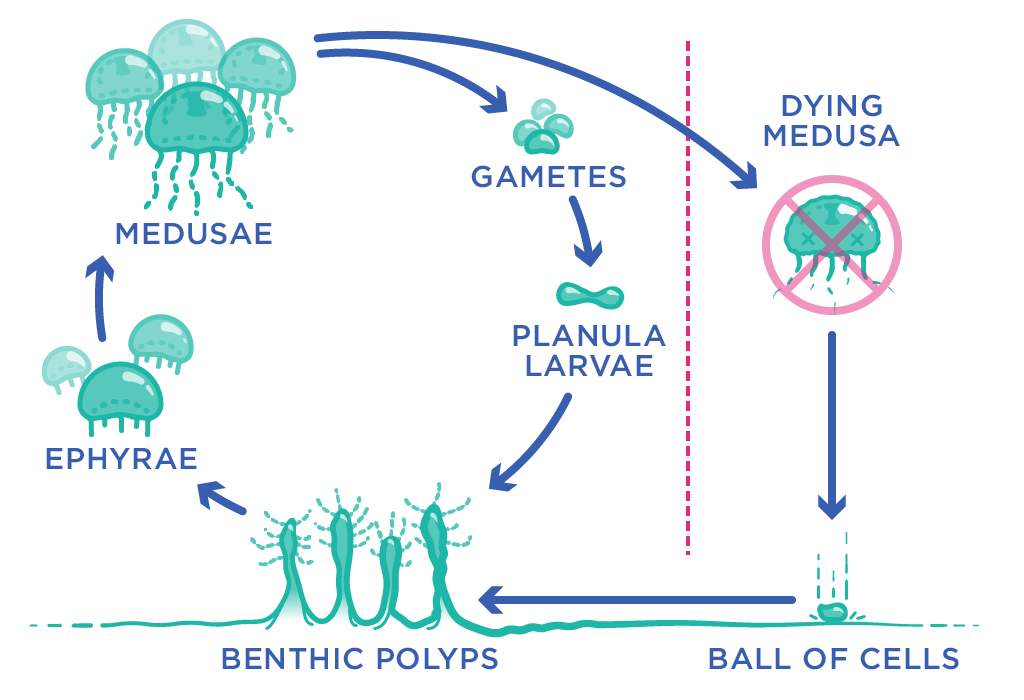
A new jellyfish life begins with a fertilised egg, which grows into a larval stage called a planula. After a quick swim, the planula latches onto a surface (like a rock, or the ocean floor, or a boat’s hull), where it develops into a polyp: a tube-shaped structure with a mouth at one end and a kind of ‘foot’ at the other. It remains stuck in place for some time, growing into a little colony of polyps that share feeding tubes with each other.
Eventually, depending on the jellyfish species, one of these polyps will form an outgrowth called a ‘bud’, or it may produce separate segments stacked on top of one another, that can then break away from the rest of the colony. This process is responsible for the next stages of the jellyfish life cycle: the ephyra (a small jellyfish) and the medusa, which is the fully-formed adult stage capable of sexual reproduction.
For most other jellyfish, this stage is the end of the line. But Turritopsis dohrnii (and possibly some other jellyfish species too) has a neat party trick: when it faces some kind of environmental stress, like starvation or injury, it can revert back to being a tiny blob of tissue, which then changes back into the sexually immature polyp phase of life. It is a bit like a butterfly turning back into a caterpillar, or a frog becoming a tadpole again.
Of course, Turritopsis dohrnii isn’t truly ‘immortal’. They can still be consumed by predators or killed by other means. However, their ability to switch back and forth between life stages in response to stress means that, in theory, they could live forever.
Hydra
Hydra look a bit similar to the polyp stage of a jellyfish (which makes some sense, given that jellyfish and Hydra are grouped together in the phylum Cnidaria): a tubular body with a tentacle-ringed mouth at one end and an adhesive foot at the other. They’re very simple animals that spend their days mostly staying in one place in freshwater ponds or rivers and using their stinging tentacles to grab any prey that happens to swim past.

Their claim to immortality? It seems as though they don’t go through senescence at all. Instead of gradually deteriorating over time, a Hydra’s stem cells have the capacity for infinite self-renewal. This seems to be thanks to a particular set of genes called FoxO genes, which are found in animals from worms to humans and play a role in regulating how long cells will live for.
In the case of Hydra’s stem cells, there seems to be an overabundance of FoxO gene expression. When researchers prevented FoxO genes from functioning, they found that Hydra’s cells began to show signs of ageing and would no longer regenerate as they did before. We still don’t know exactly how it all works, but we do know that these genes clearly play an important role in maintaining Hydra’s endless youthfulness.
Not-quite-immortal lobsters
Lobsters also do not experience senescence. Unlike Hydra’s reliance on particular genes, however, their longevity is thanks to them being able to endlessly repair their DNA.
Normally, during the process of DNA copying and cell division, the protective end-caps on chromosomes, called telomeres, slowly get shorter and shorter, and when they are too short, a cell enters senescence and can no longer keep dividing.

Lobsters don’t have this problem thanks to a never-ending supply of an enzyme called telomerase, which works to keep regenerating telomeres. They produce lots of this enzyme in all of their cells throughout their adult lives, allowing them to maintain youthful DNA indefinitely.
Telomerase is not unique to lobsters. It is present in most other animals, including humans, but after passing the embryonic life stage, levels of telomerase in most other cells decline and are not sufficient for constantly re-building telomeres.
Unfortunately for lobsters though, there’s a catch: they literally grow too big for their own shells. Lobsters continually grow larger and larger, but their shells can’t change size, meaning a lifetime of ditching too-small shells and growing a brand-new exoskeleton each time. That takes a fair amount of energy. Eventually, the amount of energy required to moult a shell and grow another new one is simply too much. The lobster succumbs to exhaustion, disease, predation or shell collapse.
Forever young?
There are many other animal (and non-animal!) species that offer tantalising glimpses into an ageless existence: the risk of dying for naked mole rats appears to not increase as they get older; the world’s oldest known non-colonial animal, a remarkably stress-resistant ocean-dwelling quahog clam named Ming, only died (accidentally) after a good 500 years when researchers dredged it up out of the ocean and wanted to find out how old it was; incredibly ancient bristlecone pines seem to function just as smoothly as much younger trees do; a particular colony of quaking aspens is considered to be about 80,000 years old … and there are plenty of other unusually long-lived species that seem to defy the passing of time.
Do they hold the key to eternal youthfulness for humans, too? We know that ageing in humans is thanks to a multitude of factors, many of which we still don’t entirely understand. Perhaps these examples from other species can shed some more light on those processes.





