Malaria-carrying mosquitoes shut down with gene drive
Mosquitoes are more than just annoying summertime pests: they are some of the deadliest animals on the planet. The tiny, blood-sucking, itch-causing mozzies are the perfect carriers for a range of nasty (and often deadly) diseases, including dengue fever, yellow fever, the zika virus and malaria.
Malaria, alone, infects hundreds of millions of people and results in hundreds of thousands of deaths every year. According to the World Health Organization, it kills a child under the age of five every two minutes. The disease is caused by tiny single-celled parasites from the genus Plasmodium, which infect human red blood cells and are spread from person to person by female mosquitoes from the genus Anopheles. Despite global efforts to eradicate malaria, progress has stalled.
But what if, rather than focusing on wiping out the disease, we could wipe out the mozzies responsible for spreading it instead? Well—thanks to research published in Nature Biotechnology, we are one step closer towards using genetic engineering techniques to do just that, with researchers causing the ‘complete collapse’ of a small population of caged malaria-carrying mosquitoes.
This is not the first time researchers have tried using CRISPR-based gene drive technology to thwart the malaria-spreading abilities of mozzies. It is, however, the first time they’ve been able to get an edited gene to spread through a population so thoroughly that, after several generations, every single mosquito has the edited gene and the population is destroyed as a result.
Genetic engineering is nothing new. A whole range of different techniques and technologies have been used over the years to edit genes in organisms to develop new medicines and better agricultural crops, for example.
But just editing a gene isn’t enough. For sexually-reproducing species, that edited gene will only be passed down to roughly 50 per cent of an individual’s offspring (assuming the edited gene is present in the egg or sperm cells). The other half will inherit the non-edited version of that same gene. After many generations, that edited gene may only be present in a tiny handful of organisms in a population.
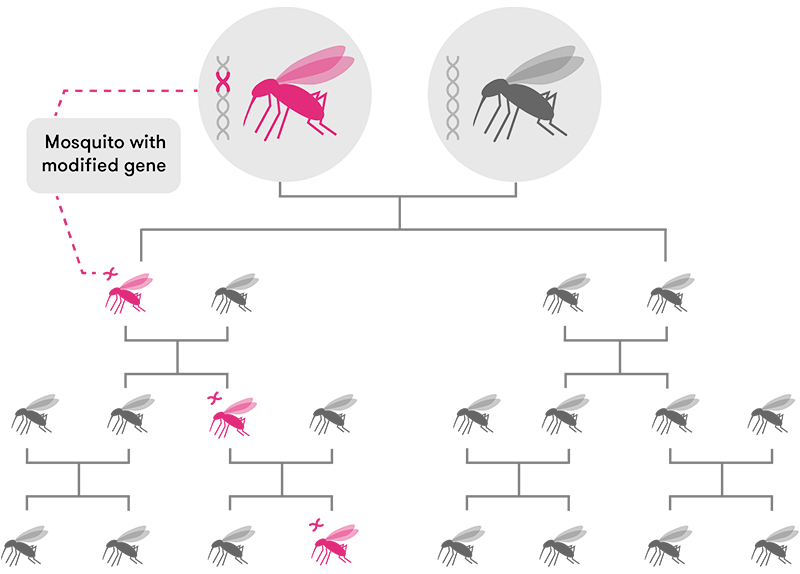
CRISPR-based gene drive technology solves this problem by ‘forcing’ the new gene to be passed on to almost 100 per cent of the offspring (in mosquitoes, at least). This means the new edited gene will spread very rapidly through a population over a few generations until most, if not all, individual organisms have that same edited gene.
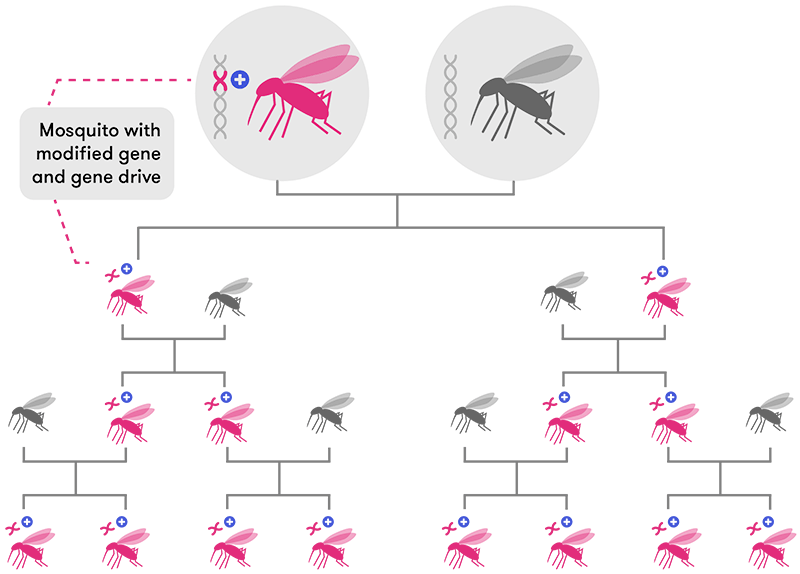
Previous attempts at using gene drives to engineer mozzie populations have only been partially successful. While the modified genes made their way into a few subsequent generations, the mosquitoes eventually developed resistance to the modified gene: that is, new mutations arose that could ‘override’ the engineered genes and halt their further spread.
Now, it seems like a solution to the problem of resistance may have been found. Researchers focused on targeting a gene (called ‘doublesex’) that controls whether a mosquito develops into a male or a female. Males with the engineered gene still developed normally but females with two copies of the gene were unable to ‘bite’ or produce eggs.
When researchers tested how well this gene spread through a population of caged mosquitoes, they found that after 7 to 11 generations, the gene was present in every individual and there were no females left that could reproduce. The population collapsed.
This doesn’t mean scientists can start tackling populations of wild mosquitoes in the real world just yet. The next step is to test out the effects of the gene drive in larger confined spaces that are much more like real conditions in the field, and with a lot more mozzies. There is still the possibility that some mosquitoes could somehow develop resistance to the gene, or that something else could affect how well the gene spreads through a much larger population under more natural conditions.
However, since this is the first time scientists have been able to cause a total population collapse in a malaria-spreading species in the lab, it’s very exciting progress in the fight against malaria.





