Why fires burn
We all know that if you gather up a bunch of dry twigs, grass and leaves and put a lit match to them, they’ll burn. Add some more sticks and bigger bits of wood and you’ve got a proper fire, ready for marshmallows. But how does fire actually work?
Fire is the result of applying enough heat to a fuel source, when you’ve got a whole lot of oxygen around. As the atoms in the fuel heat up, they begin to vibrate until they break free of the bonds holding them together and are released as volatile gases. These gases react with oxygen in the surrounding atmosphere. This chemical reaction causes a lot of heat, so much heat, in fact, that it can keep driving the reaction—as long as there’s enough fuel and oxygen still present, the reaction will become self-sustaining. The actual flames of the fire are the release of some of the heat energy as light.
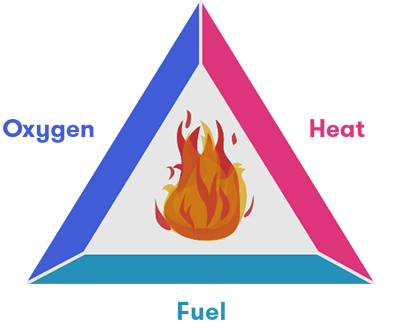
These components have led to the development of the ‘fire triangle’ of fuel, oxygen and heat. Remove any one of these and fire cannot sustain itself.
Why does water put out fire?
The primary role water plays in putting out a bushfire is cooling it down so there’s no longer enough heat to sustain the fire. When you pour water onto a fire, the heat of the fire causes the water to heat up and turn into steam. This is a very energy-intensive reaction, and it sucks away the heat (which is a form of energy) of the fire. This leaves the fire without enough energy to keep burning.
Less significant is the role water can play in ‘smothering’ a fire, depriving it of the oxygen that it also needs to burn.





