Cancer cells light up with chemistry
You could call it a light-bulb moment. In 2006, Professor Kate Jolliffe’s colleague happened to glance at a whiteboard over her shoulder. The molecule she had drawn looks like it could interact with cell membranes, he suggested.
It took a decade of hard work, but Jolliffe has turned this spark into a molecular ‘light bulb’ that can light up dying cells.
“It can be used in any application where researchers want to see if cells are dying,” says Jolliffe, “for example to monitor whether a cancer drug is having the desired effect and killing the cancer cells.”
How to switch on a molecular ‘light bulb’
The ‘light bulb’ can pinpoint dying cells by spotting phosphatidylserine on the outer surface of the cell membrane. In healthy cells, it is a building block of the membrane’s inner layer. A cell displaying phosphatidylserine on its surface is like waving a white flag—a known indication of cell death.
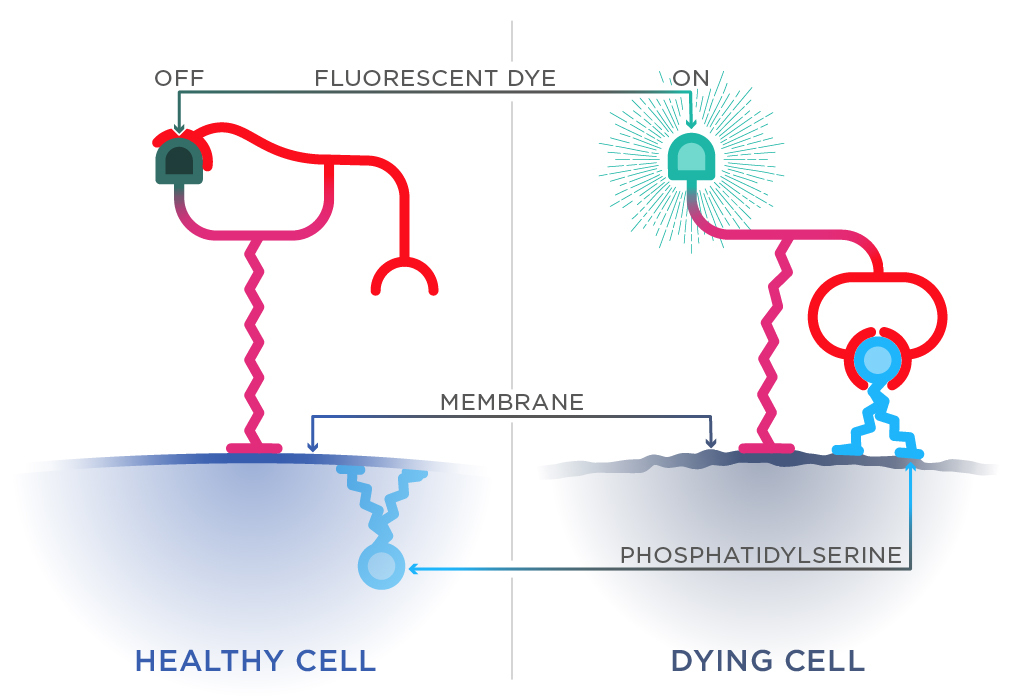
Jolliffe’s molecular light bulb consists of three components:
- a slot for phosphatidylserine
- a fluorescent dye molecule
- a hook to anchor the sensor to the cell membrane.
Fluorescent molecules absorb one colour (wavelength) of light and emit a different colour. We can’t see the UV light that glow-in-the-dark paints absorb, but we can see the visible light they emit. That’s fluorescence!
Jolliffe’s light bulb only ‘switches on’ when it scoops up phosphatidylserine. To do this, it must adjust its hold on the dye molecule. Freed from its restraints, the dye molecule can fluoresce. Scientists can simply check their cells under a microscope to see if any light up.
Picking out anions from chemical soups
The light bulb is one in a series of molecular architectures designed by Jolliffe using the ‘toolbox of organic chemistry’. Many of them are built for detecting anions: molecules or atoms with extra electrons, giving them a negative charge.
“The species that we look at, anions, are present everywhere. They're in industry, they're in the environment, and they're in biology,” she says.
They’re in our waterways. Measuring nitrates and phosphates helps us monitor the health of the ecosystem. While both the elements nitrogen and phosphorus are important for the environment, too much of either can result in algal blooms with potentially disastrous consequences.

They’re also in our blood. Chloride is an important electrolyte, and monitoring blood electrolyte levels can be used to help diagnose serious conditions like high blood pressure, liver disease or even heart failure.
Natural environments and biological fluids are both complex chemical soups. Designing a molecular structure to capture a specific anion isn’t enough—Jolliffe’s structures also need to have a strategy in place to deal with interference from other anions.
It’s a tough problem to solve—not just for Jolliffe, but for all chemists. “Trying to design a system that will bind to a specific anion in water, for example, is still something that we’re really not very good at doing,” she says.
But the journey is filled with delight. “One of the beauties of chemistry and synthetic chemistry is the ability to create a molecular structure that nobody's ever made before, which is kind of neat,” says Jolliffe.
Chemistry beyond the molecule
Chemists generally study single molecules. Supramolecular chemists like Jolliffe are more interested in the interactions between two or more different kinds of molecule. It’s the “chemistry beyond the molecule”, as 1987 chemistry Nobel Laureate Jean-Marie Lehn famously described (PDF, 4.6 MB).
Studying these interactions teaches chemists how molecules could be coaxed into forming complex structures spontaneously, with little interference from the chemists themselves. This is called self-assembly.

It’s a process mastered by the most sophisticated chemist of all: nature. Essentially, all biological organisms—including you—are just a very complex assembly of molecules.
“Supramolecular chemists effectively try to make small, simple systems that mimic the functions of nature’s complex machinery,” says Jolliffe.
And the chemists are getting better at it. The 2016 Nobel Prize in Chemistry was awarded to supramolecular chemists who created the world’s smallest machines.
Jolliffe was recently elected a Fellow of the Australian Academy of Science in recognition of her contributions to Australian science.





