Mining the Moon
Space mining. It’s the stuff of science fiction for now, but it could be a real possibility very soon—and our very own Moon could be a prime target.
So, what does the Moon have that makes it such a tantalising prospect for space miners? Does it have lots of big, hidden deposits of gold, or diamond, or rare metals? While the Moon does harbour deposits of many materials of great value to us here on Earth, there are two other things that have sparked a lot of interest: water and helium.
Using water for rocket fuel
If you thought your last overseas trip was a bit pricey, spare a thought for those involved in space exploration. Space travel is incredibly expensive and resource-intensive. A rocket launched from Earth needs to carry with it all the fuel that it needs to escape Earth’s strong gravitational pull and then get to its destination—and sometimes to get back to Earth again after that. The more fuel you need to carry with you, the more weight you add to your rocket, which means you’ll need even more fuel to push it through space.

If only there were places for rockets to refuel somewhere along the way … like, perhaps, a service station orbiting the Moon.
Scientists recently found ‘definitive evidence’ that water ice exists on the Moon. This was an exciting discovery for several reasons, particularly in the context of a future where people could potentially live on the Moon. Such local water could be used for people to drink, bathe and grow plants.
Perhaps a more immediate use for this water, though, is to make rocket fuel. Water molecules are made of hydrogen and oxygen, both of which are super useful for rocket propellants. The molecules can be split apart by running an electric current through the water (electrolysis), yielding hydrogen and oxygen, which can then be stored as liquids, ready to fuel a rocket.
This could allow rockets to leave Earth with just enough fuel to make it to the Moon, where they could then re-fuel before moving on to the next destination. Alternatively, fuel from the Moon could be transported to a fuel depot in low Earth orbit, allowing rockets to dock for re-fuelling closer to home. Either way, it means a more efficient use of fuel and energy that could potentially allow spacecraft to travel a lot deeper into space and lower the costs of space exploration activities.
Using helium-3 for energy
It’s one thing to think about the resources we need for space exploration—but we also need to find new solutions for powering our lives right here on Earth. We need to find new, economical ways of generating energy that don’t rely on finite resources (such as fossil fuels) and are less damaging to the environment.
Could there be a solution on the Moon? Potentially—and it involves helium, an element with a whole bunch of uses that are far more impressive than filling party balloons or making your voice squeaky. For instance, helium could theoretically be used to generate massive amounts of energy through nuclear fusion reactions.

To use helium for fusion power, you need an isotope (a form) of helium called helium-3, which has one less neutron in its nucleus compared to ‘normal’ helium. Fusing two of these atoms together (or fusing a helium-3 atom with an atom of deuterium, an isotope of hydrogen) under very high temperature and pressure yields an enormous amount of energy. In fact, nuclear fusion is what powers the Sun and other stars—it’s energy generation on a much bigger scale compared to any current technologies here on Earth!
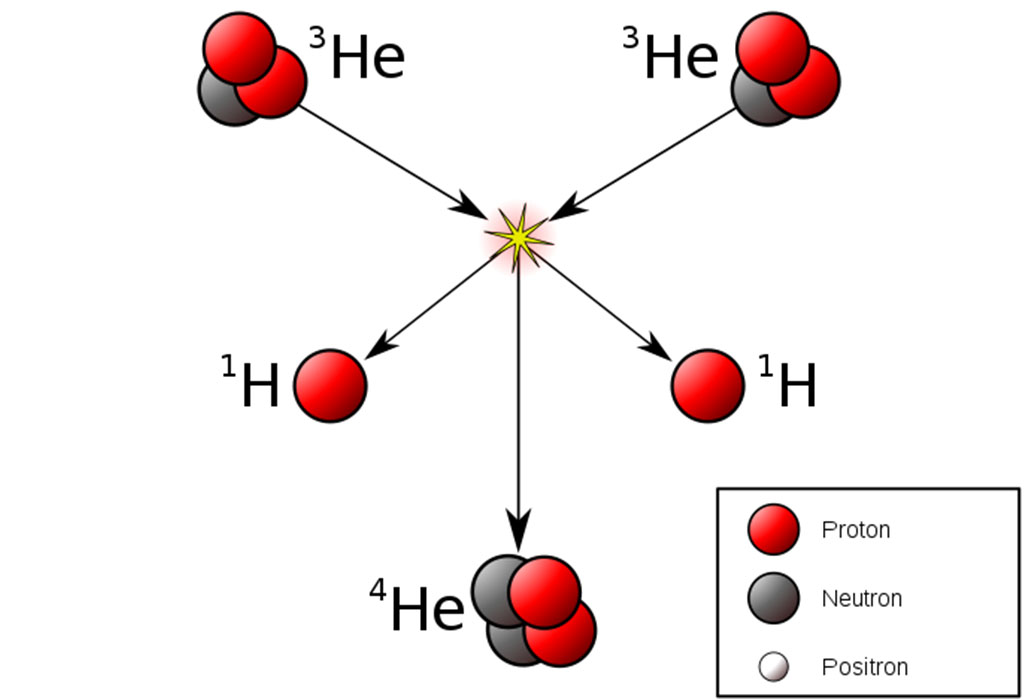
Nuclear fusion power is still a theoretical technology rather than a practical reality. Despite plenty of ongoing research and development, it is unlikely that we would see working fusion power generation technology for at least another ten years. There are still some major hurdles to overcome, such as finding a way to contain the high-temperature materials involved and dealing with the neutrons that are released in most fusion reactions that can damage the walls of a reactor.
With helium-3, neutron release isn’t a problem. There are no other radioactive by-products to deal with, either. This is a massive advantage when compared to some other experimental fusion reactions, such as fusing deuterium and tritium (another hydrogen isotope), or with existing nuclear fission processes.
Helium-3 travels through space via the streams of charged particles flowing from the Sun, called the solar wind, but Earth’s magnetic field prevents these particles from reaching us. There is virtually no magnetic field on the Moon, so its surface gets bombarded with all these charged particles (including helium-3). This makes the layer of rock and dust on the Moon’s surface a good candidate for obtaining several materials that are far less abundant on Earth.
If we could find a cost-effective way to mine the Moon’s surface for helium-3, transport it back to Earth, and then use it to generate energy via nuclear fusion, we could generate vast amounts of energy with no radioactive by-products and no greenhouse gas emissions. That’s still a very long way off into the future for now, though.
Who should mine the Moon?
Despite the challenges, harnessing the Moon’s resources is enough to inspire private companies and space agencies from multiple countries to take mining the Moon very seriously. Which raises two very important questions: who owns the Moon, and what effect would Moon-mining have on Earth?
Don’t worry: mining all that helium won’t cause the Moon to deflate and fall out of the sky. Nor will mining operations have enough of an impact to affect the mass of the Moon in any major way—even if the Moon lost just 1 per cent of its total mass, this still would not significantly affect its orbit, or the gravitational pull on Earth’s oceans that causes high and low tides.
As for ownership, the 1967 United Nations Outer Space Treaty says that no nation can claim ownership of the Moon. However, that doesn’t necessarily prevent private companies from claiming portions of the Moon as their own commercial property. It’s an ethical conundrum that space law experts and ethicists have not solved yet—but it seems we might need to start thinking of a solution soon.





